DNA Damage Response
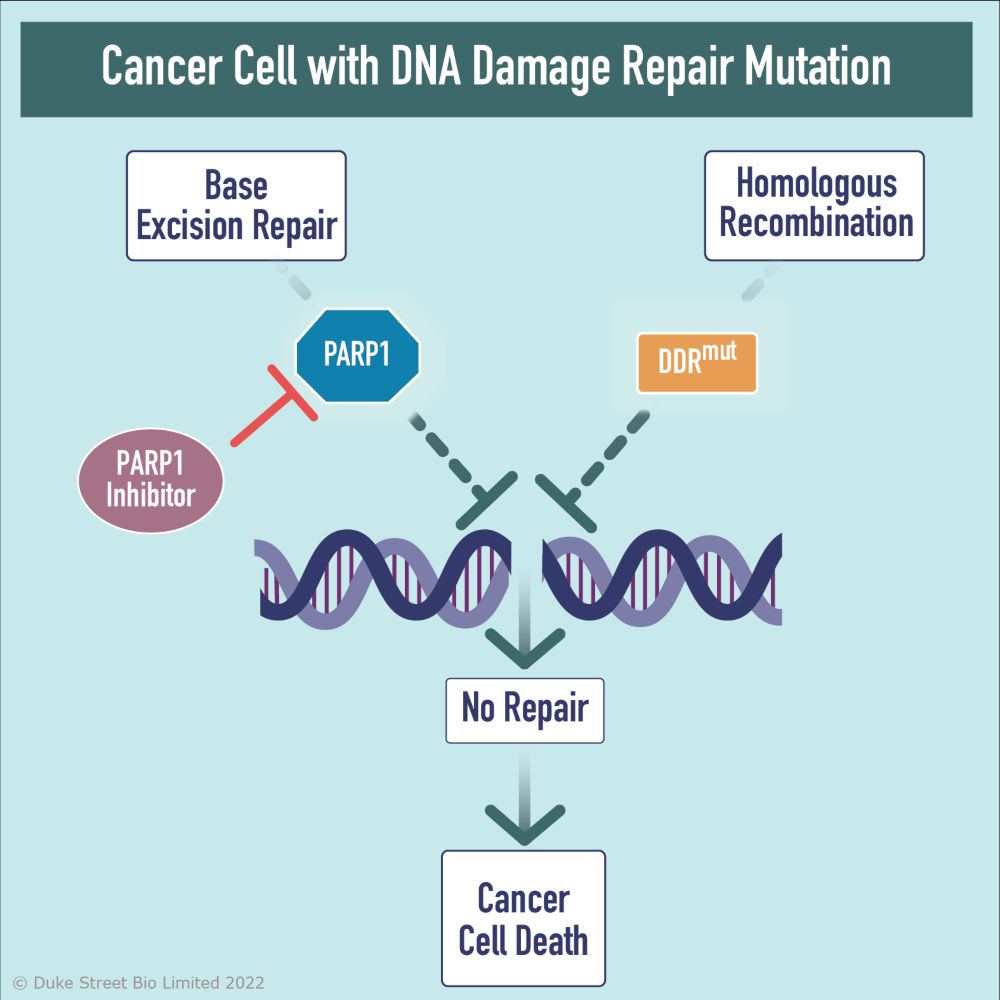
Cells utilise multiple mechanisms, collectively known as the ‘DNA Damage Response’ (DDR) to detect, signal and repair DNA damage in order to maintain genomic integrity. Defects in DDR genes such as BRCA1 & 2 play critical roles in driving genomic instability, carcinogenesis and tumour growth.
Inhibitors of core proteins involved in the DDR such as the poly (ADP-ribose) polymerase, PARP1, have been utilised to significant therapeutic effect in tumours harbouring mutations in DDR pathways, such as homologous recombination. These mutations render tumours exquisitely susceptible to inhibition of PARP1. This concept of ‘synthetic lethality’ has underpinned the approval of PARP1 inhibitors in BRCA-mutated ovarian, breast, prostate and pancreatic cancer.
Highly Selective PARP1 Inhibitors
Duke Street Bio has developed inhibitors highly selective for PARP1 over PARP2. This second-generation approach offers a significant opportunity to 1) radically enhance therapeutic index, 2) enable additional precision medicine / combination approaches with chemotherapy and targeted agents and 3) expand the addressable patient population to those whose tumours harbour additional DDR defects.
Duke Street Bio’s development candidate, DSB2455, is a potentially best-in-class, potent, CNS-penetrant PARP1 inhibitor with profound selectivity over the closely related enzyme PARP2. This molecule is currently being investigated in a Phase 1a/1b trial in participants with advanced malignancies.
Delivering life-saving cancer treatments to improve patient outcomes.
